In The Thick Of It: Accelerating The Mixing And Delivery Of High-viscosity Injectables
As featured in On Drug Delivery
High-viscosity and difficult-to-mix injectables are becoming more prevalent as pharmaceutical pipelines fill with novel biologics and incorporate a long-overdue focus on patient experience through less frequent, self-administered treatments.
“As pharmaceutical companies work to bring their molecules to market, they are faced with a difficult trade-off: extend development timelines to investigate lower-viscosity formulations that may be compatible with existing autoinjectors or revert to large-volume doses administered via on-body devices or IV infusions.”
While increasingly promising, complex injectables – including biologics, long-acting injectables, lyophilised formulations and other advanced therapies – present challenges throughout the value chain, from formulation to filling to administration.
One particularly difficult challenge is handling the significantly increased viscosities inherent to many of these formulations. As pharmaceutical companies work to bring their molecules to market, they are faced with a difficult trade-off: extend development timelines to investigate lower-viscosity formulations that may be compatible with existing autoinjectors (not good for commercial success) or revert to large-volume doses administered via on-body devices or intravenous (IV) infusions (not good for patients). Some pharmaceutical companies may even choose to defer or cancel development programmes due to viscosity challenges despite significant unmet patient needs. Windgap Medical has a solution to this conundrum with its proprietary large-volume dual-chamber (LVDC) autoinjector platform that enables subcutaneous (SC) and intramuscular administration of high-viscosity injectables.
THE CASE FOR – AND CHALLENGE OF – SELF-ADMINISTRATION
Patients and caregivers often prefer SC injections over IV injections due to a lower risk of infection and other adverse reactions, as well as an increased ability to self-administer, reducing time spent in clinical settings.1
To achieve the 1–2 mL administration volumes typically required for SC injection, biologics must be formulated at high concentrations, which exponentially increases protein-protein interactions and, therefore, formulation viscosity.2 These high concentrations may also lead to stability challenges, such as aggregation and denaturation, when in solution. One method of addressing this issue is lyophilisation, which keeps the drug product in a more stable powdered form until the time of injection.
Long-acting injectables (LAIs) come with additional viscosity challenges. LAIs offer patients convenience and safety compared with traditional injectables, as fewer treatment sessions are required and the pharmacokinetic profile between treatments may be more uniform.3 These formulations often use high-molecular-weight carriers to achieve successful delivery and controlled release, which naturally increases the viscosity of the combined solution. LAIs are commonly lyophilised to minimise the stability concerns that stem from fragile molecules, carrier aggregation and premature drug release.
The good news is that pharmaceutical companies are not alone in this effort. As they work on novel drug formulations to address the viscosity challenges of mixing, dosing and injecting, device companies such as Windgap Medical are designing solutions from a different angle.
A BETTER PATIENT EXPERIENCE LEADS TO BETTER PATIENT OUTCOMES
Patient adherence can be a significant challenge for high-viscosity drugs. Readying viscous drugs for administration often demands complex preparation routines. As these drugs are frequently left in, or converted to, powdered form for storage and transportation, mixing is required to reconstitute them at the point of care. This may be done via manual shaking, swirling, tapping or a combination of these methods. User instructions often specify extended preparation times to overcome natural human variability in speed, vigour and movement, which presents a significant barrier to adoption and access.
“The gas pressure can be customised with minimal design changes to meet the force requirements for different target injection volumes, times and viscosities.”
In the case of highly viscous formulations, it becomes impractical to consider mixing a solution that behaves like a syrup or honey. For example, UCB’s (Brussels, Belgium) Cimzia® (certolizumab pegol), a commercial treatment for Crohn’s disease, can take up to 30 minutes to fully reconstitute.4 Even in the simplest cases, there is a risk of incomplete reconstitution due to orientation dependencies and lack of a well-defined endpoint. Incomplete reconstitution can have severe consequences for patients, including immunogenicity and incomplete dosing.5
Once ready for injection, these thicker doses require more time, larger needle diameters or even both to deliver the same volumes. This increase in time and discomfort can decrease a patient’s willingness to take the injection again. However, a BD & Eli Lilly study showed that, when controlled for needle dimensions and flow rate, perceived injection site pain was lower at higher viscosities.6 This is a promising finding, suggesting that a device design capable of accommodating high viscosities without changing the needle gauge improves patient comfort over low-viscosity formulations.
As viscosities increase, higher forces are required to maintain tolerable delivery times. However, many devices on the market today are still spring-based systems, which are prone to breakage and malfunction if too much force is applied. The working range of these systems is further limited by the decreasing force output of spring power over the duration of the injection.
A PROMISING SOLUTION TO A LONG-TERM CHALLENGE
With thousands of new therapies entering clinical trials each year, it is ever more critical to design novel delivery devices that simplify the administration of these life-changing drugs for both pharmaceutical companies and patients.
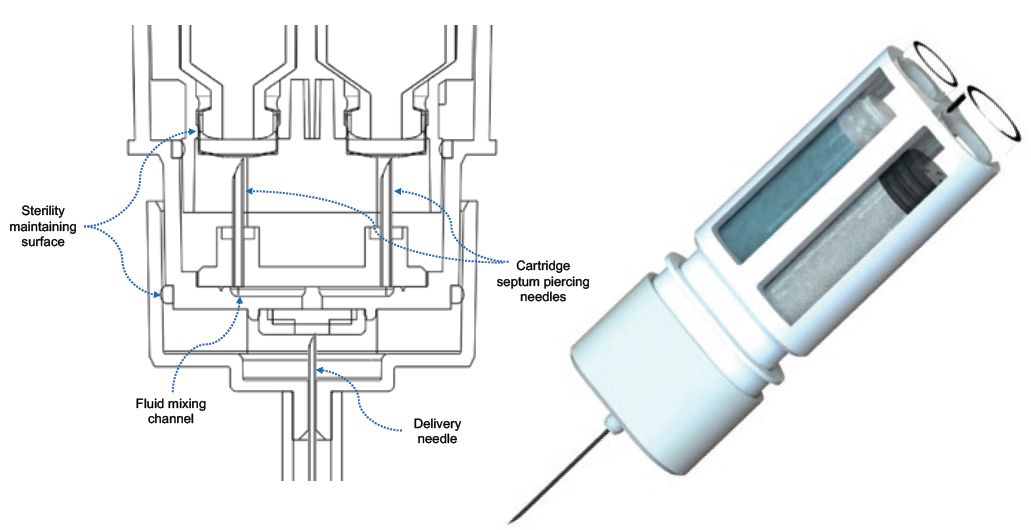
Figure 1: On the left, a cut-away schematic of Windgap’s proprietary mixing and delivery hub. On the right, the novel side-by-side configuration of standard, single-chamber cartridges.
The novelty of Windgap’s LVDC platform stems from its innovative arrangement of off-the-shelf primary drug containers (PDCs), as shown in Figure 1. The architecture features side-by-side nesting of two single-chamber cartridges with Windgap’s proprietary mixing and delivery needle hub. The side-by-side nesting permits the use of readily available, ISO-compliant cartridges compatible with industry-standard filling methods for powder and liquid products while maintaining a compact, easy-to-handle form factor. Cartridge sizes from 1 to 5 mL can be accommodated. Windgap’s LVDC products are gas powered to enhance functionality when managing both high viscosities and large-volume injections.
The device platform is currently being used to develop solutions for automated mixing and delivery of lyophilised compounds or co-therapies in three (or fewer) steps. To achieve this, device activation causes septum-piercing needles to puncture both cartridges simultaneously. This dual puncture creates a closed fluidic connection between the two chambers, transferring the liquid to the powdered drug cartridge for initial mixing. The device automatically regulates the release of stored gas to reciprocate the solution back and forth between the two cartridges and complete the mixing process, removing the need to shake or swirl. The device-controlled mixing reduces the number of user steps and effort required to prepare and administer a lyophilised or powdered drug.
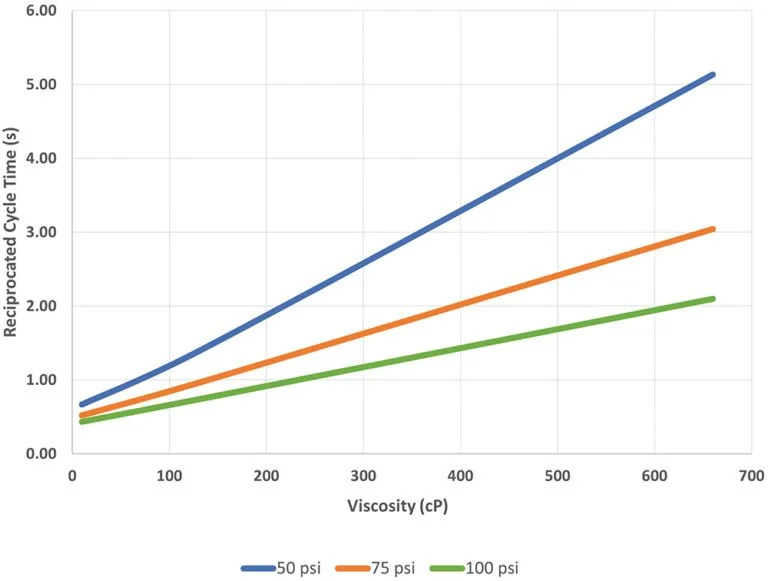
Figure 2: The time in seconds to complete one mixing cycle for a 3 mL dose is shown with respect to applied pressure for a range of viscosities. Data include the time required to actuate valving and pressurise the system at the beginning of each test.
In a recent feasibility study conducted in partnership with a top-five pharmaceutical company, Windgap evaluated the LVDC platform’s performance across a range of viscosities. This study tested mixing times for 1, 2 and 3 mL fluid volumes across a range of viscosity standards using off-the-shelf 3 mL cartridges; the standards were then delivered through 27G, 29G and 31G needles. The reported time for one reciprocated mixing cycle is the time required for the plunger of one cartridge to be fully depressed and then returned to its starting position as the fluid travels back and forth via the mixing hub.
Figure 2 highlights the LVDC’s ability to mix formulations up to and beyond 500 cP. When driven at 100 psi, the device completes a mixing cycle for 3 mL of 500 cP test fluid in less than two seconds, with faster times possible for smaller volumes and lower viscosities. If managing shear stress is more critical than minimising mixing time for a given application, the mixing rate can be tuned to mix the product more gently and prevent shear-related degradation.
Table 1 provides a glimpse of what is possible at smaller volumes and lower, more common viscosities. The mixing time in seconds is reported for the full range of tested volumes and viscosities at set pressures. A 1 mL volume of 10 cP liquid can be mixed in less than half a second per cycle with 50 psi and, more impressively, one mixing cycle for 3 mL of 100 cP liquid can be completed in less than a second with 75 psi.
“The controlled, reciprocated mixing between the side-by-side cartridges presents an exciting opportunity to shift … to device-controlled mixing with a quantifiably validated endpoint.”
Table 1: Time in seconds to complete a single mixing cycle given a fluid volume and viscosity (applied gas pressure shown in parentheses for each viscosity). Data includes the time required to actuate valving and pressurise the system at the beginning of each test.
Previous feasibility studies with other pharmaceutical partners have indicated that the LVDC system continues to function effectively when operated at the higher pressures required to administer formulations beyond 5,000 cP. In all studies, the mixing hub did not inhibit the delivery flow path and injection times were similar to those demonstrated by other gas-based devices for a given needle gauge, applied pressure and dose; for example, the LVDC delivers 3 mL of 10 cP liquid through a 29G SC needle in 9.9 seconds at 100 psi of pressure. This expanded working range makes the platform a reliable and efficient choice for future drug pipelines. The gas pressure can be customised with minimal design changes to meet the force requirements for different target injection volumes, times and viscosities.
PUTTING FORMULATION DEMANDS AND PATIENT NEEDS SIDE BY SIDE
The Windgap team incorporates a patient-centric approach to device development, prioritising human factors engineering early in the design process to develop a robust patient experience while also solving technical challenges. With the push of a button, LVDC users – patients, caregivers and healthcare professionals – can activate the internal gas-powered mechanisms that then regulate mixing and administration of the contained therapy.
Table 2: Key benefits of the LVDC autoinjector platform.
When the device is in its fully automatic configuration, treatment may be delivered in just three steps – initiate mixing, remove cap, inject – with little human force required. Furthermore, the LVDC platform has demonstrated acceptable injection times without the need for a painfully large needle. Its performance with higher concentrations and viscosities opens the door for lower-volume injections and a broader range of therapies to be converted from IV to SC injection.
Additionally, the controlled, reciprocated mixing between the side-by-side cartridges presents an exciting opportunity to shift from manual mixing with subjective evaluation of completion to device- controlled mixing with a validated, quantifiable endpoint after a pre-determined number of mixing cycles. Whether reconstituting a dry powder or mixing two liquids, such a shift promises more consistent mixing outcomes and reduces the risk of error from ill-defined instructions to “shake”, “swirl” or “tap” at the point of care. This is just one example of how Windgap empowers patients by designing with both the end use and end user in mind.
As shown in Table 2, the LVDC platform is well-suited to adapt to the rising tide of challenges, viscosity or otherwise, flooding through the industry’s drug pipelines.
Conclusion
Windgap Medical is on a mission to inject simplicity into complex drug delivery. Its LVDC platform addresses the challenges of traditional autoinjector approaches and large-volume, high-viscosity formulations. The company seeks to “solve beyond the solution” to develop highly innovative drug delivery devices that leverage its patented technologies. Windgap Medical welcomes partnerships with the pharmaceutical industry for custom development programmes based on the LVDC platform or its rescue medication (ANDI®) platform.
REFERENCES
Bittner B, Richter W, Schmidt J, “Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities”. BioDrugs, 2018, Vol 32, pp 425–440.
Rosenkranz T, Kalman S, “Enabling Stable, High-Viscosity Injectable Drug Products with New, Excipient Combinations”. Pharma’s Almanac: Nice Insight’s Content Community, Mar 12, 2022.
Gonella A et al, “Long-acting injectable formulation technologies: challenges and opportunities for the delivery of fragile molecules”. Expert Opin Drug Deliv, 2022, Vol 19(8), pp 927–944.
“How to prepare and administer in-office injection”. CIMZIA® (certolizumab pegol), 2023.
Kulkarni S. “Overcoming Long Reconstitution Times of High Concentration Lyophilised Protein Formulations”. University of Connecticut Doctoral Dissertations, 2019.
Berteau C et al, “Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance”. Medical Devices (Auckland, NZ), 2015, Vol 8, pp 473–484.
Some statements are forward-looking. Unless specifically stated, these devices are not approved for sale in the US or the EU.